Energy and Entropy: Winter-2026
Lab 4: Rubber Band : Due Day 26 W9 D3
- Rubber band lab - Ethan
S0 5460S
In this lab, we will measure the tension of a rubber as a function of length. By making this measurement at different temperatures, we will be able to use a Maxwell relation to determine the change in internal energy \(U\) and entropy \(S\) for a rubber band that is stretched at fixed temperature.
Materials:
- Rubber band
- Glass tube
- Stopper with hook
- Vernier force meter (better than spring-meter)
- Thermometer
- Several clamps
- Boiling water
- Ice
- Pan
Background
A small amount of work done on a rubber band with tension \(\tau\) and length \(L\) is \begin{equation} {\mathit{\unicode{273}}} W = \tau dL. \end{equation} This follows naturally from the definition of work as force dotted with distance---provided one takes into account the sign convention for tension, which is opposite that of pressure. Thus the thermodynamic identity is: \begin{equation} dU = TdS + \tau dL \end{equation}
We could use the thermodynamic identity directly, but since we are working at constant \(T\), it is more helpful to consider the Helmholtz free energy \begin{equation} F \equiv U - TS \end{equation}
We will use \(F\) in the analysis below.
The setup
You will stretch your rubber band between a force meter and a hook in the stopper in the bottom of a pipe. You will attach the rubber band to the force meter by means of a short chain. The rubber band will be completely immersed in water when the pipe is filled with water. This chain will enable you to conveniently change the length of the rubber band, by changing the link of the chain that is passed through the hook on the force meter.
During the experiment you will adjust the temperature of the rubber band by pouring boiling water, or ice-cold water, into the pipe. You will need to take measurements of the force as a function of length for several different temperatures. You will use a temperature probe inserted into the top of the pipe to measure the temperature of your water---and thus the temperature of your rubber band.
Collect data
You should take data of the tension as a function of length for different temperatures, over as wide a range as possible. If you can, take the same data more than once---for instance, return to your original temperature after making several other measurements---you may be able to determine whether there has been any drift.Tasks and questions
- Tension vs. length From you own data, plot the tension versus length for a low temperature (ice cold water) and a high temperature (near 60 or 70\(^\circ\)C).
Data from literature Plot the following data*:
length (cm) \(\tau\) (N) \(\tau\) (N) @ 1.4\(^\circ\)C @ 67\(^\circ\)C 24.4 0.82 1.08 28.0 1.75 1.90 31.6 2.38 2.53 35.2 2.87 2.96 38.8 3.25 3.35 *Roundy and Rogers, Am. J. Phys. 81 20 (2013).
Notice that the data from Roundy and Rogers shows higher tension at higher temperature. Does your data also show this trend? If your data does not follow this trend, please use the Roundy and Rogers data for the rest of this analysis.
- Tension vs. temperature On a new graph, plot tension versus temperature for each of your lengths. Put all the data onto a single graph. For each length, what value of \(\left(\frac{\partial {\tau}}{\partial {T}}\right)_{L}\) is the best fit to your data?
Maxwell relation Starting from the defintion of Helmholtz free energy, \(F\), for a rubber band show that \begin{equation} \left(\frac{\partial {S}}{\partial {L}}\right)_{T}=-\left(\frac{\partial {\tau}}{\partial {T}}\right)_{L} \end{equation}
Hint: It will be helpful to start with the equation for \(F\), zap with \(d\), and compare with the appropriate overlord equation.
- Change in \(F\), \(S\) and \(U\). Pick a temperature (for example 1.4\(^\circ\)C), and the shortest length and longest length (for example 24.4 cm and 38.8 cm). Use your experimental data to answer the following questions as accurately as you can:
- What was \(\Delta F\) for this isothermal stretch?
- What was \(\Delta S\) for this isothermal stretch?
- What was \(\Delta U\) for this isothermal stretch?
- What was \(Q\) for this isothermal stretch? (Was this energy transferred in, or out, of the rubber band?)
- What was \(W\) for this isothermal stretch? (Was this energy transferred in, or out, of the rubber band?)
- Stat mech model of a rubber band
S0 5460S
Polymers, like rubber, are made of very long molecules, usually tangled up in a configuration that has lots of entropy. As a very crude model of a rubber band, consider a chain of \(N\) links, each of length \(l\) (see Figure). \(N\) is very large. Imagine that each link has only two possible states, pointing either left or right. The total length of the rubber band is the net displacement from the begining of the first link to the end of the last link.
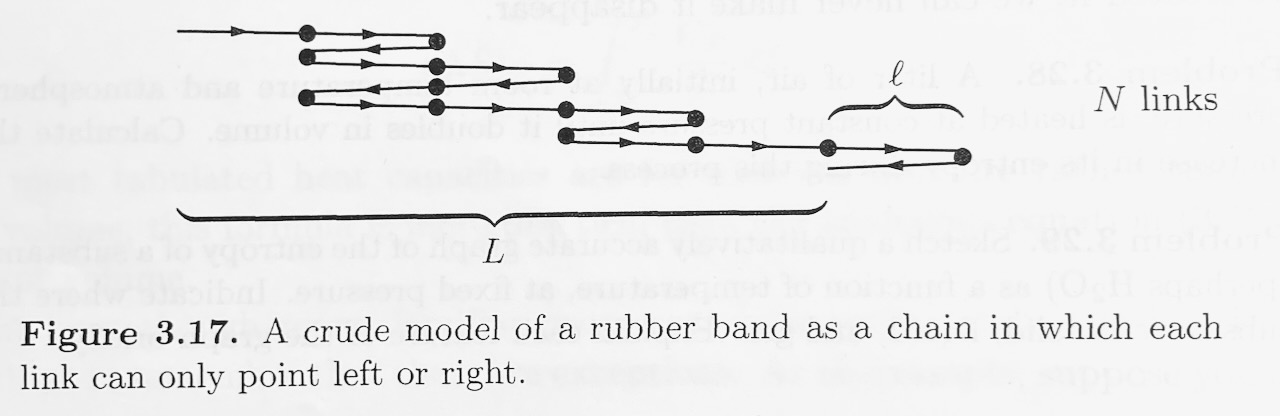
- Find a differentiable expression for the entropy of this system in terms of \(N\) and \(N_R\), where \(N_R\) is the number of links pointing to the right. For guidance calculating multiplicity, see Schroder's text book, \(\S\)2.1 "Two-State Systems". To write a smooth function \(S(N,N_R)\), where \(N\) and \(N_R\) are treated as continuous variables, you will need to use the idea that \(N\) and \(N_R\) are very large, and Stirling's approximation: \begin{equation} lnx! \approx xlnx-x \text{ , when \(x \gg 1 \)}. \end{equation}
- Write down a formula for \(L\) in terms of \(N\) and \(N_R\).
- What is the thermodynamic identity for this system (you will have to replace \(-pdV\) with something else).
- Using the thermodynamic identity, express tension in terms of a partial derivative of entropy. From this expression, compute the tension in terms of \(L\), \(T\), \(N\) and \(l\).
- Show that when \(L \ll Nl\), the tension is directly proportional to \(L\) (Hooke's law)
- Discuss the dependence of the tension force on temperature. If you increase the temperature of the rubber band, does it tend to relax or tighten.
- Suppose that you hold a relaxed rubber band in both hands and suddenly stetch it. Would you expect its temperature to increase or decrease? Explain.